Independently on the type of glass we are going to produce, or which kind of furnace we are going to install, the final quality of our glassware product might be affected by a huge reject due to dimensional instability or cosmetical defects, which are both linked to the quality of the glass melt in terms of physical and chemical homogeneity, thermal stability, aging of contact glass refractories and turnover time of the melt inside refractory tank (furnace).
However, the temperature profile is taken under control by continuous monitoring with a series of thermocouples installed on precise spots along furnace and distributors. Also the pull rate is continuously monitored, directly and indirectly by the production ratio, but for what concern turnover inside of the furnace, homogeneity of the melt and how it is distributed inside from the loader to the throat, and if there are created some stagnant zone or short circuit, those parameters, together with chemical analysis of the glass, would not be enough in the majority of the situations.
Just to evaluate the behavior of the glass melt in the furnace (related to furnace type, shape, aging, profile) a powerful tool is the so called “Residence Time Distribution Curve”. As the name well explain a Residence Time Distribution Curve (RTDC from now) is exactly a curve which trace a concentration of a certain element (tracing agent) in a series of glass samples collected along the process (generally cold end samples).
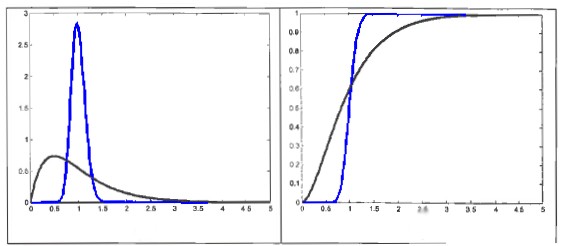
Fig1: on the left RTDC piston flow(blue) and mixed(black). On the rigt Transition Function
First of all it is important to create a RTDC by using a tracing agent (Ta) to add to molten glass. As Ta it is possible to use a chemical element not comprising in the batch pack, in a correct quantity to be detectable, but also to not affect glass properties avoiding
- modification in flux inside the furnaces,
- problems in forming zone,
- variation of typical characteristics of final product (transition metals are to avoid due to colouring properties, also in low concentration; in addition Iron is usually already present around 100 – 150 ppm as baseline, coming from stainless steel and raw materials contamination).
Other plus should be that the relative available raw material have to be as simple as possible (oxides best choice).
Depending on detection methods it is possible to use alkali or earth or metallic oxides such as Sr2O, ZnO, MgO (when not used as modifier or were Dolomite is not a batch component) or SnO2; all of them are relatively cheap and easily melted, do not change glass colour, are easily detectable by chemical analysis and they do not give any contribution in glass properties variation in the quantity needed.
ZrO2 it is not a good choice because it is hard to melt and could give inclusions revealed as defects in cold end than rejected, and secondarily it is the main component of contact glass refractories so it would be not effective as Ta. Another good choice it is CeO2 that wherein the glass, show an intense fluorescence activity under special lamp, which help to obtain a RTDC in a fast way. For what concern the best quantity to use to obtain a valuable RTDC, it is necessary to consider, the detection limit of available analitycal technique and than the total quantity of molten glass bath; a general acceptable starting point should be 10 kg of oxide / 100 tons of melt that correspond to 100 ppm (part per million) if all melt is conditioned immediately by all the Ta.
Ta have to be introduced directly in the loader as empty as possible just to prevent unwanted dilution and/or delay. Since introduction of Ta in the loader, it is necessary to start to collect sample in cold end by a scheduled plan, labeling each samples with day, time, line. Frequency of sampling is not fixed but is decided based on experience (low frequency till first show, high frequency to the peak, low frequency again is one possibility); the same for what concern total time of RTDC, even if it is always a good choice at least 3 turnovers time (depends on total molten glass and pull rate). Each of those choices could affect the resolution of the RTDC, of course the deep of possible analysis and the reliability of results. It is clear that for all the time of RTDC test. the pull rate should have to be maintained as stable as possible.
Last advice is related to cullet: for all RTDC test time it is important to not reuse the cold end cullet to prevent cross contamination and results mystification. In theory it is also possible to use a combination of Ta (two is more common) especially when there are more than one loader, to tracing different loading zones flux and effects.
Once we have obtained RTDC it is necessary to analyze and “translate” the results to recover any available information. Tipical RTDC has a shape as following:

Fig2: some examples of realistic RTDC
The shape give immediately an information on the grade of mixing of molten glass: ideal piston flow is typical for unmixed process (quantic flow) where the “packet “ of batch (and than glass) flows along the tank without mixing. The shape associated to a well mixed process, is more likely close to second one; in this case the sample relative concentration of Ta is lower and also the peak is less pronounced, and it is exactely due to mixing process (convective back flow, etc.) that promote the conditioning of all the molten bath.
The main 2 parameters to consider to well interpretate the RTDC are:
– time of first appearance of Ta, the so called First Show or Minimum RT
– time of the appearance of maximum concentration of Ta, the so called Peak or Maximum RT
Relative position of this 2 points, comparing along the campaign could give a first qualitative information regarding furnace mixing process and flows. To go deeper in the analysis it is important to consider other factors such as:
– Normalized time. To have a repeatable and comparable RTDC time has to be normalized considering so called geometrical resident time or turnover time (τ) obtained considering the total amount of molten glass in the tank and the pull rate for example 100 tons of melt by 2 tons/h of pull rate means a τ = 50 h. By converting time in % of τ it is possible recover much more information from our RTDC as for example if there is some preferential track in the tank, some stagnant zones, more flows (more spikes) and also if the real process is a combination of flows (piston + mixed) and in which ratio.
– FWHM (Full Wide at Half Maximum). Last parameter I would suggest, is not a common one but it is based on my experience and I have verified how it might be interesting. The over mentioned FWHM is a typical parameter used in spectroscopy or Physics and Chemistry to evaluate a peak or a “spike” in optical or electronic analysis. This number is obtained considering on the curve (spike) the 2 points that show approximately the half height of the maximum and measuring the delta in terms of time. This number is the so called FWHM that give information on the shape of the spike and consequently on the “resolution” of the signal. Applying this concept to RTDC, it is clear how well It is possible to evaluate what happen before the Peak (with First Show principally) but also after the appearance of the maximum concentration of Ta. This parameter can give to Technologyst all the informations regarding how the eventual spike is thin and resolute (piston flow ideal) or if the peak is broad and how is the magnitude of this effects. It is possible to better understand preferential track associated to stagnant zones, mixing grade, delay, or some unwanted cross contamination.
In effect, process to obtain RTDC is a sort of “Chromatography”, where the stationary phase and mobile phase are still the molten glass. Ta is the component that carried by mobile phase which become in contact with stationary one. The interactions and mixture between Ta and glass determine the shape of RTDC. In the same way the chemical affinity between component and stationary phase in Chromatography define the detection time and peak (spike) shape: in RTDC the “affinity” is translated in “mixing grade” and is strictly correlated to melting process, technical parameters and to the furnace situation (design and aging).